Warning Signs on Supplements Sold in California
Proposition 65 requirements for Dietary
Supplements sold in California


2. What measures has PRL taken to protect customers from lead contamination in our products?
If you live in California, you have probably noticed the warning signs we have placed on certain dietary supplements in order to comply with Prop 65, a law enacted only in California. PRL takes great pride in the quality of our dietary supplements which are made only from healthy, natural ingredients. Since many of these natural ingredients are of botanical origin, the absorption of miniscule amounts of lead (Pb) due to the inherent metal levels present in water and soil during plant growth is not unusual.
PRL has taken extreme precautions to ensure that our products contain the lowest possible amount of naturally occurring heavy metals by requesting that our manufacturer does the following: careful sourcing, selecting and diligent testing, using state-of-the-art analytical techniques such as inductively coupled plasma mass spectrometry (ICP-MS). ICP-MS is one of the most sensitive techniques currently available on the market for measuring heavy metal content and can detect as low as 0.01 ppb (0.01 microgram per kg) of heavy metals. All of the raw materials in our products are screened for heavy metal content before use in formulations. Every production batch of each final product is tested again (after manufacture) to confirm that our products meet or exceed the FDA and USP heavy metal thresholds.
What is Proposition 65?
Proposition 65 (formally titled “The Safe Drinking Water and Toxic Enforcement Act of 1986”) is a California law that was passed by voters in 1986 [1].
Proposition 65 requires businesses to provide warnings to Californians about significant exposures to chemicals that can cause cancer, birth defects or other reproductive harm. These chemicals may be present in products that Californians purchase, in their homes or workplaces, or released into the environment.
Warning signs are required in places (gas stations, enclosed parking facilities, dental offices, hotels, restaurants, amusement parks, etc.) and on products (alcoholic beverages, passenger and off-highway vehicles, furniture products, food and dietary supplement products, etc.) to warn consumers of the presence of these potentially harmful chemicals.
Copyright 2021 Quantum Nutrition Labs
Warning signs are now required in many different businesses in California, such as in enclosed parking garages, amusement parks and on food and dietary supplement products.
The Prop 65 chemical list, which is required to be updated at least once per year, has grown to include approximately 900 chemicals since it was first published in 1987. Chemicals found on the list range from synthetic chemicals to elemental impurities which can be naturally occurring in organic ingredients.
Proposition 65 does not require warning signs posted on your tap water, although according to California State law [2&3], the action level of 15 ppb (mcg/L) set for lead (Pb) exceeds the daily intake level allowed by the FDA in food by over 2.5 times. If we take this one step further and examine how much lead an average person might consume on a daily basis, we find a staggering level of 40 mcg lead intake/day, which is 80 times what Prop 65 allows for dietary supplements (0.5 mcg lead intake/day) and almost 7 times the FDA recommended limit of 6 mcg lead intake/day.
All PRL products that bear the Prop 65 warning signs have lead levels that are well within the FDA/USP acceptable limits for lead (except for two products, see below). However, we are still required to place a warning sign on products due to Prop 65 requirements.
ICP-MS is an extremely sensitive and accurate analytical technique used for determining lead content, but it cannot distinguish between “bound lead” and “free lead” in product samples. It can only detect total lead. This is an important distinction because “bound lead” is not typically absorbed by the body whereas “free lead” may be absorbed.
One PRL product, Medi-Clay-FXTM, contains bentonite clay derived from the earth. Bentonite clay has a unique crystalline structure and an overall negative charge on its surface [9]. Any lead ions (Pb2+) present in bentonite are trapped inside these crystalline structures and are therefore well


bonded with surrounding atoms. When this product is ingested and travels through the digestive system, the lead ions are not expected to be released and absorbed. In fact, the clay molecules have an unusually large surface area with such a strong negative charge that they will act like strong magnets to attract positively charged lead ions and remove them from the system.
These clay molecules are far too big to pass through the lining of the digestive tract and into the blood stream and other tissues, and therefore cannot be absorbed. Clinical toxicology data [10] had shown that clay molecules will simply pass through the digestive system, “cleaning house” as they move along, removing lead and other heavy metals from the body. This acts as a detoxifying agent and is expected to capture absorbed and trapped heavy metal ions which have entered the body as soluble forms from toxic materials via other ways, such as food or paint [11].
Therefore, although Medi-Clay-FXTM contains lead values slightly above the Prop 65 limit of .5 mcg/day (5.9 mcg/day and 6.3 mcg/day respectively), it is mostly as “bound lead” and is not expected to be absorbed.
If the typical American diet alone may exceed the Prop 65 levels every day, how meaningful are the lower limits for supplements? What does this prove?
This shows how stringent and impractical these levels are to achieve for all dietary supplements and cosmetics, especially products made with ingredients derived from nature.
The quality of PRL’s products far exceeds the industry standards even if the Prop 65 guidelines require that we add warning stickers to certain products.
We manufacture our products to ensure that robust standards of quality, purity, composition and identity are met in accordance with guidelines set by the FDA and USP. PRL tests all nutritional products for heavy metal levels with extensive quality control procedures.
PRL strictly adheres to cGMPs, USP and FDA regulations. Since it is virtually impossible to manufacture natural
botanical dietary supplement products without trace amounts of lead due to the inherent lead levels present in water and soil, each finished product is also analyzed for heavy metal levels.
Every dietary supplement product produced at PRL is released for human consumption after meeting PRL Quality Standards that meet or exceed federal safety standards.
PRL controls and monitors the source of our raw materials throughout the manufacturing process until the product leaves our facility and goes to the customer. Superior quality and transparency to our customers are the foundation of PRL.
3. Is Prop 65 (lead exposure) a True Concern?
The concern for exposure to elevated levels of lead is valid; however, the limits set by Proposition 65 in the state of California are unrealistically low for dietary supplements and cosmetics, especially when compared to other daily forms of lead exposure. Relative to the heavy metal lead, Prop 65 requirements have raised the allowable amount to a level that no dietary supplement industry can achieve.
If we drink the recommended amount of water or eat the recommended 2-3 servings of vegetables a day, we could be consuming lead amounts over the Prop 65 levels.

4. How Does the Prop 65 Lead Limit Compare to Acceptable Limits
Set by the FDA and USP?
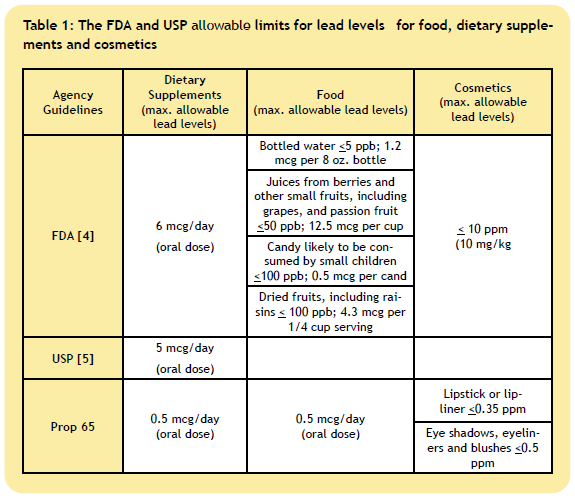


5. How are the permitted daily exposure limits calculated according to the FDA, USP and WHO?
Extensive research studies have been conducted on lead (Pb) toxicity and human health. Based on the results of these studies, health organizations (FDA, USP and WHO) have set an extremely stringent Permitted Daily Exposure (PDE) limit [6]. PDE is the maximum acceptable daily intake of an elemental impurity.
These daily exposure limits are derived from the No-Observed-Adverse- Effect Level (NOAEL), or the Lowest- Observed-Adverse-Effect Level (LOAEL) taken from the most relevant animal studies extrapolated to the effect on human health.
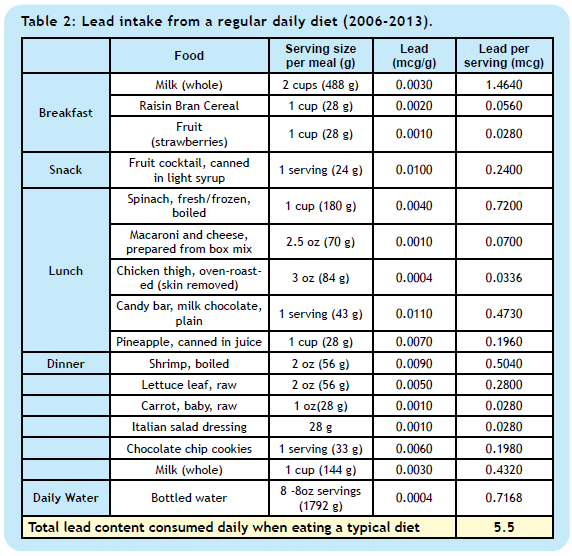
6. How much lead do we ingest in our regular daily diet?
Total lead intake from eating a standard diet calculated based on lead content published in the US FDA Total Diet Study (Market Baskets 2006 – 2013) [7] is shown in Table 2.

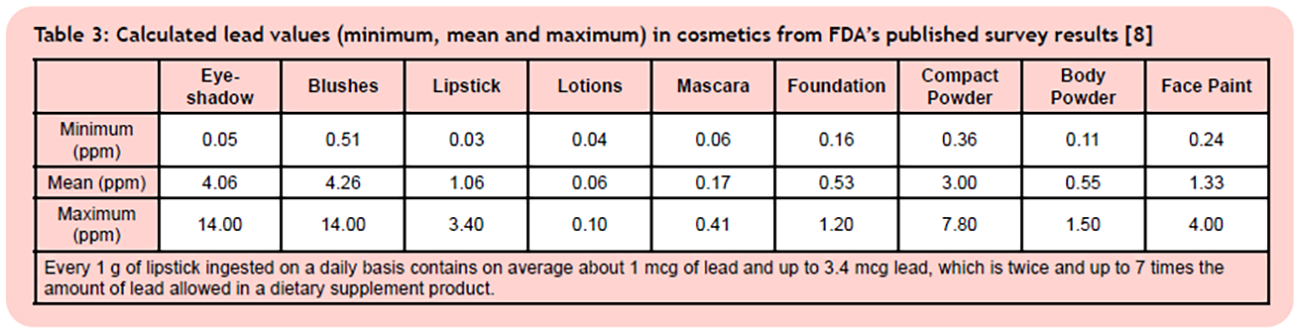
7. How Much Lead Gets Applied on Our Lips and Skin During a Daily Makeup Routine?
The lead content found in typical cosmetics applied to lips and skin are shown in Table 3.

References:
1. https://oehha.ca.gov/proposition-65
2. https://www.calwater.com/waterquality/lead-water/
3. https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/documents/leadandcopperrule/implementing_lead_education_provisions_lcr.pdf
4. https://www.fda.gov/
5. https://www.uspnf.com/
6. https://www.fda.gov/downloads/drugs/guidances/ucm371025.pdf
7. https://www.fda.gov/downloads/food...totaldietstudy/ucm184301.pdf
8. https://www.fda.gov/Cosmetics/ProductsIngredients/PotentialContaminants/ucm4
9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2895274/
10. https://www.ncbi.nlm.nih.gov/pubmed/18568297
11. https://www.globalhealingcenter.com/natural-health/6-health-benefits-bentonite-clay/
R21-0122



